We consume various kinds of products from time to time. Whenever you have an headache, probably you may look for pharmaceutical products in a drug store. One of the pharmaceutical products used quite frequently in our daily lives is the Pain Killer, called Panadol.
There was a mini-research project organised for the Testing and Certification, and Life Science students in CIE. There are two parts in the project. First of all, the students had the opportunity to go through the whole process in the quantitative determination of active ingredients in Panadol tablet.
In the second part, the active ingredient, paracetamol, was used to prepare solutions of various concentrations and added to Zebrafish embryo at ambient conditions. The physiological effect of paracetamol on the heart rate was monitored.

Fig. 1 Weighing using the analytical balance
In the chemical analysis of the tablets, students tried using simple apparatus like spatula, glass pipettes and analytical balance. After the tablet sample was weighed by a 4-decimal place analytical balance (Fig. 1), it was ground into a white powder using pestle and mortar (Fig. 2).

Fig. 2 Grinding of the tablet into powder

Fig. 3 Solvent used to extract paracetamol
It is not an easy job as the tablets are rather big and pretty hard to be made into the powder form. Then, with the help of an ultrasonic bath, the active ingredient was extracted into an organic solvent (Fig. 3) for subsequent analysis by High Performance Liquid Chromatography (HPLC) (Fig. 4).

Fig. 4 High Performance Liquid Chromatograph
Since there are a lot of chemical components in the tablet, we employed the HPLC to separate various chemical components based on the different migration rate of different components inside the HPLC column (the heart of separation).
This is an internationally recognised analytical method being used in analysis of paracetamol in tablets. We have established a proper calibration curve for paracetamol standard solutions (Fig. 5a and 5b). Based on the equation of the calibration and tablet weight, the amount of paracetamol per tablet was found to be quite close (<5%) to the amount shown on the label of Panadol.

Fig. 5a Students operating the HPLC for analysis of paracetamol.
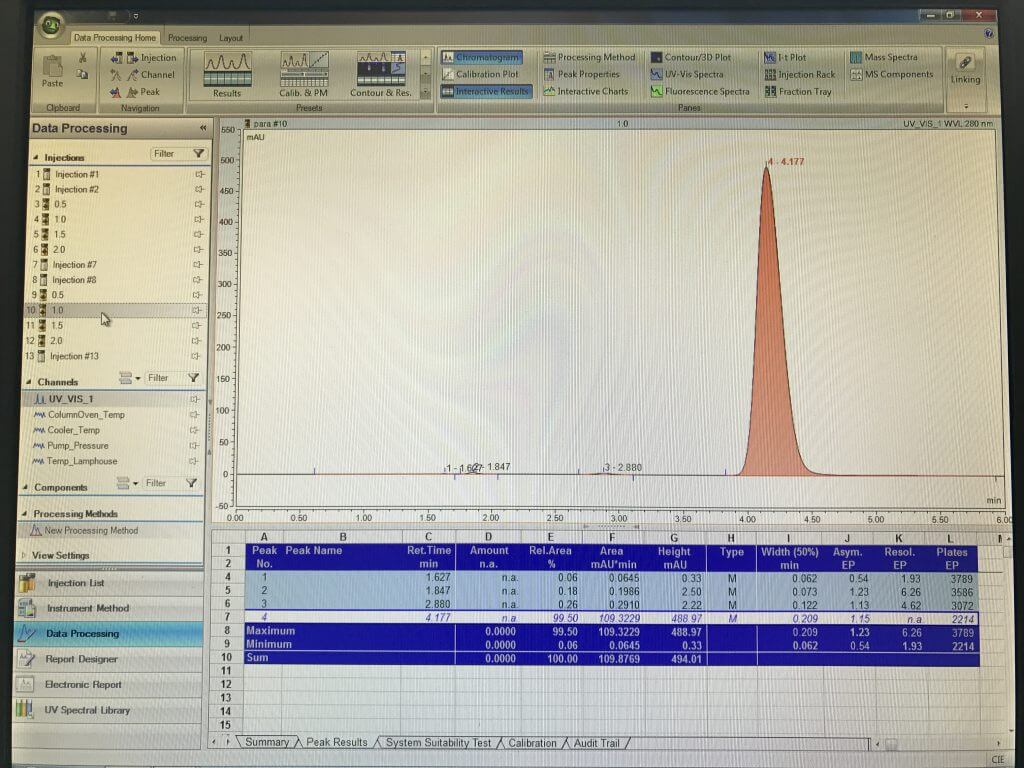
Fig. 5b data obtained from HPLC
In the second part, the students made proper dilution of the given paracetamol solution into different concentrations (Fig. 6). The diluted solutions were cautiously added separately into the aqueous media containing Zebrafish embryo (Fig 7a and 7b).

Fig. 6 Proper dilution of paracetamol solutions

Fig. 7a Addition of paracetamol solutions into Zebrafish embryo

Fig 7b Zebrafish embryo in an aqueous medium containing paracetamol
The behaviour of Zebrafish embryo was observed under the microscope. At regular time interval, the heart beat rate were determined by the students using simple measurement and calculation.

Fig. 8 Observation under the microscope
At last, the students did an oral presentation (Fig. 9) of their original data and findings with high confidence before they received a certificate to recognize their effort put in the mini-project.

Fig. 9 Oral presentation of experimental results from chemical and biological analysis